Research interest
Animals engage social behaviors for their own survival and the survival of their species. Social behaviors include sexual behaviors for reproduction, nurturing behaviors of parents, and aggressive behaviors to protect territories from intruders. The mode and frequency of such social behaviors vary greatly depending on sex, because the brain controlling social behaviors differs between sexes. Understanding sex differences in the brain is important for understanding how animals attempt to preserve themselves and their species. In humans, sex differences in the brain are related to gender identity and sexual orientation. Human society comprises not only by heterosexual men and women, but also by people representing a wide variety of gender identities and sexual orientations. We believe understanding the sex/gender of the brain is essential to build a richer society with sexual diversity. In our laboratory, we are conducting research with the aim of revealing the mechanisms that create sex differences in the brain, and the diversity of these phenomena by using mammalian and avian animal models.
Research topic 1: the formation mechanisms and physiological roles of sexually dimorphic nuclei in mammals
The brain contains sexually dimorphic nuclei that manifest sex differences in morphology and function. Factors that are important for the formation of sexually dimorphic nuclei in rodents are: (1) androgens secreted from the testes during the perinatal period, (2) steroid hormones (i.e., testicular androgens and ovarian estrogens) secreted from the gonads during puberty, and (3) sex chromosome genes that are expressed in the brain. Of these factors, perinatal testicular androgens are considered to play the most important role in the sexual differentiation of the brain. The brains of genetic females can be masculinized by exogenous androgen treatment during the perinatal period, while the brains of genetic males can be feminized by inhibiting perinatal testicular androgens. Furthermore, the structures and functions of sexually dimorphic nuclei may differ between individuals of the same sex owing to differences in the amount and timing of gonadal steroids that act on the brain. We are conducting research to clarify the mechanisms by which sexually dimorphic nuclei are formed under the influence of gonadal steroids. Additionally, we are investigating the roles of sexually dimorphic nuclei in the control of social behaviors.
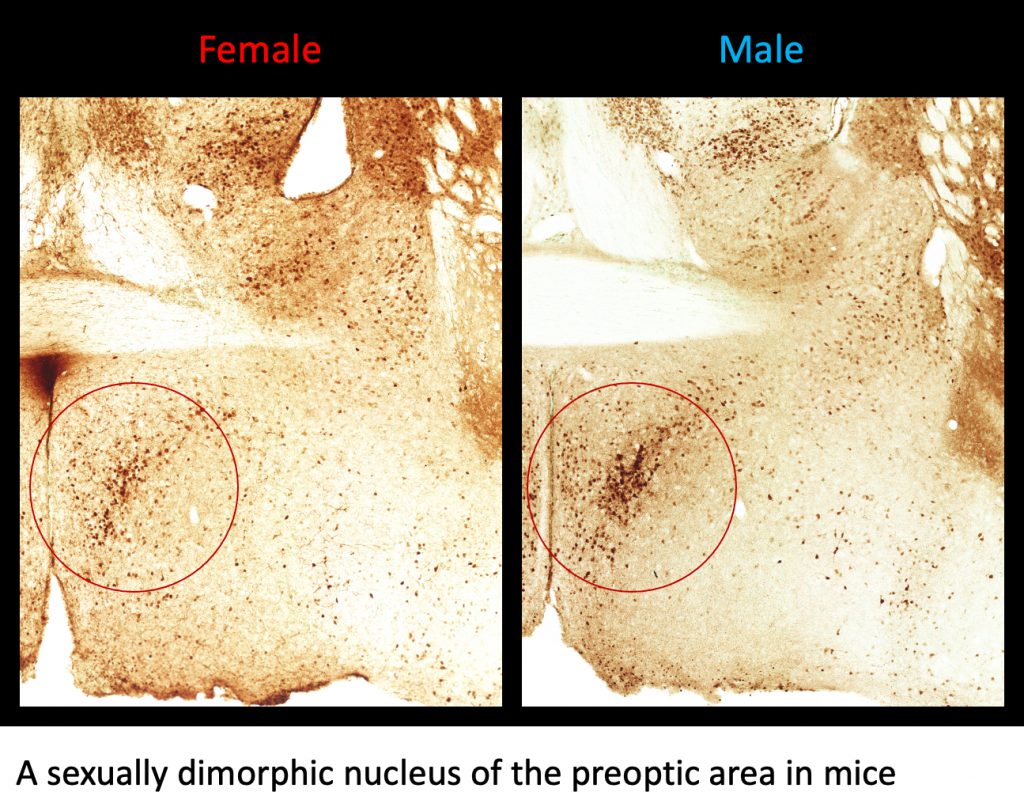
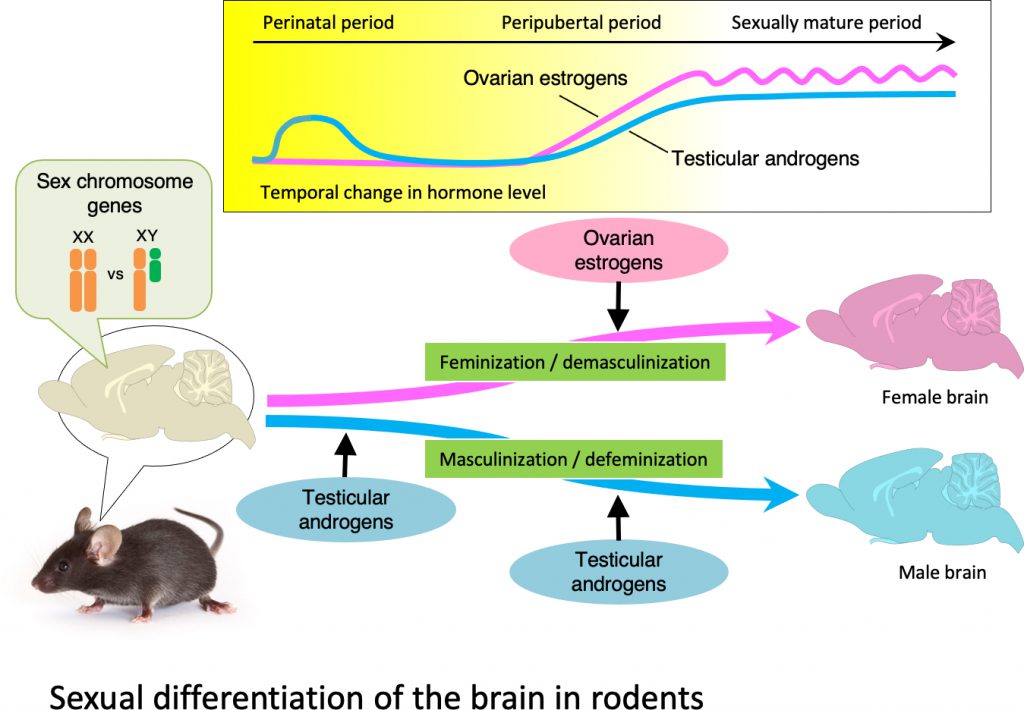
Research topic 2: the action mechanisms of ovarian estrogens of female embryos for the sexual differentiation of the brains in birds
The genetic sex of an animal is determined by the combination of sex chromosomes. Mammals born with two X chromosomes are female, and individuals born with one X chromosome and one Y chromosome are male. However, birds born with two Z chromosomes are male, and those born with one Z chromosome and one W chromosome are female. The gonadal hormones that are important for sexual differentiation in the brain also differ between mammals and birds. Androgens secreted by the developing testes are important in mammals, while estrogen secreted by the embryonic ovaries plays an important role in birds. We are conducting research to clarify how estrogen secreted from the ovaries of female bird embryos works to feminize and demasculinize the brains of birds. It is a mystery why the mechanisms of sexual differentiation differ between mammals and birds. We aim to solve the mystery by exploring and comparing the mechanisms of sexual differentiation in the brains of both birds and mammals.
Publications
- Morishita M, Arai H, Ueda C, Arai Y, Ishii H, Tsukahara S: Sex difference in vasoactive intestinal polypeptide neural projection in the limbic system of the mouse brain. Endorcinology, 166, bqaf150, doi: 1210/endocr/bqaf150, 2025.
- Arai Y and Tsukahara S: A male-dominant cell group expressing calbindin-D28K and androgen receptor in the mouse preoptic area requires postnatal testicular androgens and histone deacetylation. Jounrnal of Neuroendocrinology, doi: 10.1111/jne.70097, 2025.
- Koiso R, Kushida C, Kanaya M, Tsukahara S: Emergence of the sex difference in calbindin D-28K neurons and the role of calbindin D-28K in clustering neurons of the preoptic area in mice. Cell and Tissue Research, doi.org/10.1007/s00441-025-03981-3, 2025.
- Morishita M, Kobayashi K, Mitsuzuka M, Takagi R, Ono K, Momma R, Tsuneoka Y, Horio S, Tsukahara S: Two-step actions of testicular androgens in the organization of a male-specific neural pathway from the medial preoptic area to the ventral tegmental area for modulating sexually motivated behavior. Journal of Neuroscience, 43, 7322-7336, 2023.
- Tsukahara S, Morishita M: Sexually dimorphic formation of the preoptic area and the bed nucleus of the stria terminalis by neuroestrogens. Frontiers in Neuroscience, 14, 797, doi:10.3389/fnins.2020.00797, 2020.
- Okino E, Morita S, Hoshikawa Y, Tsukahara S: The glutamatergic system in the preoptic area is involved in the retention of maternal behavior in maternally experienced female rats. Psychoneuroendocrinology, 120, 104792. doi: 10.1016/j.psyneuen.2020.104792, 2020.
- Morishita M, Koiso R, Tsukahara S: Actions of peripubertal gonadal steroids in the formation of sexually dimorphic brain regions in mice. Endocrinology, 161, 6, 1-17, 2020.
- Nakashima S, Morisita M, Ueno K, Tsukahara S: Region-specific effects of copulation on dendritic spine morphology and gene expression related to spinogenesis in the medial preoptic nucleus of male rats. Psychoneuroendocrinology, 108, 1-13, 2019.
- Maejima S, Abe Y, Yamaguchi S, Musatov S, Ogawa S, Kondo Y, Tsukahara S: VGF in the medial preoptic nucleus increases sexual activity following sexual arousal induction in male rats. Endocrinology, 159, 12, 3993-4005, 2018.
- Kanaya M, Morishita M, Tsukahara S: Temporal expression patterns of genes related to sex steroid action in sexually dimorphic nuclei during puberty. Frontiers in Endocrinology, 9, 213, doi: 10.3389/fendo.2018.00213, 2018.
- Morishita M, Maejima S, Tsukahara S: Gonadal hormone-dependent sexual differentiation of a female-biased sexually dimorphic cell group in the principal nucleus of the bed nucleus of the stria terminalis in mice. Endocrinology, 158, 10, 3512-3525, 2017.
- Moe Y, Kyi-Tha-Thu C, Tanaka T, Ito H, Yahashi S, Matsuda KI, Kawata M, Katsuura G, Iwashige F, Sakata I, Akune A, Inui A, Sakai T, Ogawa S, Tsukahara S: A sexually dimorphic area of the dorsal hypothalamus in mice and common marmosets. Endocrinology, 157, 12, 4817-4828, 2016.
- Tsukahara S, Kanaya M, Yamanouchi K: Neuroanatomy and sex differences of the lordosis-inhibiting system in the lateral septum. Frontiers in Neuroscience, 8, 299, doi: 10.3389/fnins.2014.00299, 2014.
- Kanaya M, Tsuda CM, Sagoshi S, Nagata K, Morimoto C, Chaw Kyi Tha Thu, Toda K, Kato S, Ogawa S, Tsukahara S: Regional difference in sex steroid action on formation of morphological sex differences in the anteroventral periventricular nucleus and principal nucleus of the bed nucleus of the stria terminalis. PLoS One, 9, 11, e112616, 2014.
- Tsukahara S, Tsuda M, Kurihara R, Kato Y, Kuroda Y, Nakata M, Xiao K, Nagata K, Toda K, Ogawa S: Effects of aromatase or estrogen receptor gene deletion on masculinization of the principal nucleus of the bed nucleus of the stria terminalis of mice. Neuroendocrinology, 94, 2, 137-147, 2011.
Contact
Shinji Tsukahara, Ph.D.
Professor
Division of Life Science
Graduate School of Science and Engineering
Saitama University
Japan
stsuka(at)mail.saitama-u.ac.jp

